Properties of Air - Air Aerodynamics Flight - Science - Grade 6

Air takes up space
Air has mass/weight
Air is affected by heat
Air exerts pressure
Air can be compressed
Air is affected by altitude

Air Takes Up Space
Take an empty ziploc bag, open it and pull it through the air like a parachute. Now close it, seal it and try to squish the bag. There’s nothing in the bag, right? Wrong. The ziploc bag is full of air.
You can also prove this by blowing up a balloon. The balloon expands because you are putting something into the balloon; air. This air takes up space, so the more air you put into the balloon, the more space it takes up. When you use a pump to blow up a football, you don’t put nothing into it, you put air into it - this air takes up space which is why the football expands.
Try it Yourself:
Air Has Mass
Place an empty balloon on a scale and weigh it. Take this same ballon and inflate it. Weigh it again. What do you see?. A really clear way to show this is to make a balance with a stick or coat-hanger suspended by a string in the middle. Tie an empty balloon on each side to prove they weigh the same. Inflate one balloon and rehang it. That side of the balance will be heavier. If air had no mass, there would’ve been no change.
Air is really quite heavy. It is just that it has always been there for you and me so we do not notice. Asking a human if air is heavy is like asking a fish if water is heavy.
Every square inch of surface on the earth has about 15 pounds of air sitting on it. (Air is piled about 100 miles high on each square inch.) Just for fun, calculate the number of square inches on the top of your head and multiply it by 15. Wow... you are holding all that up!?!?
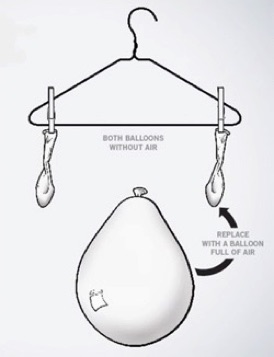
Try it Yourself:
Air Exerts Pressure
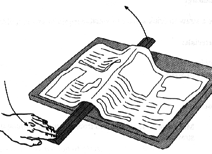
Take a metre stick and lay it on a table. Unfold a full page of a newspaper and lay it flat over the metre stick. Push down on the other end of the metre stick. What happens? Why can’t you lift a super-light piece of paper? Air exerts pressure (in all directions).
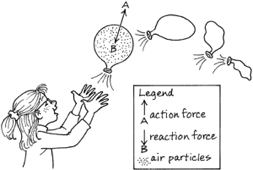
The air above the paper pushes down on it (pressure). This pressure is what makes the paper lay flat on the table - it’s being pushed down. Even though they’re too tiny to see, all the molecules of air in the atmosphere above your head weigh something. And the combined weight of these molecules causes a pressure pressing down on your body of 10,000 kg per square metre (10,000 kg = 22,000 lbs). This means that the mass of the air above the 0.1 square metre cross section of your body is 1,000 kg, or a tonne.
If you tried to lift a small car, you’d definitely notice it, so why don’t we notice that there’s a tonne of air pressing down on us? Well, the air exerts this force in all directions, so as well as pushing down on us, it also pushes up and balances out the force on our bodies so that we don’t collapse.
Video Explanation
Try it Yourself:
Air is affected by Temperature
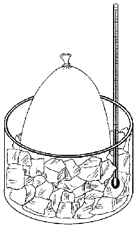
Take a balloon and place it over the top of a pop bottle (2L is best with a little bit of water in it). Observe the size of the balloon now (@ room temperature). Now place it in a freezer for 10 minutes, remove it and observe size of balloon. Now take the bottle and hold it in a baking dish of almost-boiling water for 10 minutes. Now let the bottle sit on the table for 10 minutes. You should now see the balloon return to the same size as it was to start.
The greater the temperature, the faster the air particles move (increasing pressure), hitting the sides of the balloon more often and harder, making the balloon inflate more. The colder the air becomes though, the slower the air particles move (lowering pressure), resulting in the same amount of air now taking up less space. This is why the beach-ball you left in the garage over night will be “smaller” in the morning that it was during the day (when it was warmer).

Try it Yourself:
Temperature inside and outside the balloon is equal meaning the air pressure is the same. The air pressure inside and outside balloon is the exact same, so there is no change.
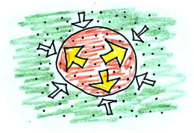
The air inside the balloon is heated, causing the air pressure to increase, making the air spread out, making it push against the sides of the balloon. The pressure inside the balloon is now NOT equal to the outside. The balloon will expand until the pressure inside the balloon (or temperature), is the same as the pressure (temperature) outside.
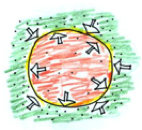
The air is cooled, decreasing the pressure. The balloon will shrink until the temperature (aka pressure) inside the balloon is equal to the temperature outside.
Air can be Compressed
Take a plastic pop bottle and with the cap off the bottle, hold you hand above the mouth of the bottle and squeeze. What do you feel? Screw the cap on tightly and squeeze again. What happens when you squeeze the bottle now? Now, fill the bottle completely with water, replace the cap and squeeze again. What do you feel now?
When you squeezed the open bottle, you forced some of the air out of the mouth. When you placed the cap on the bottle and squeezed again, there was no place for the air to go, but you were able to squeeze the bottle together. In other words, you were able to compress (or squeeze together) the air inside the bottle. However, when you filled the bottle with water and capped it, you could not squeeze the bottle very much at all because you could not compress the water inside.
Gases such as air can be compressed, but liquids such as water, cannot be compressed.

Try it Yourself:
Air is affected by Altitude
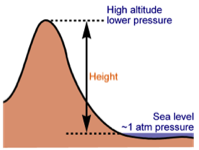
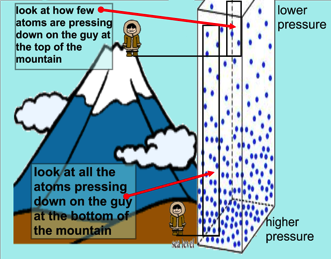
The higher you are, the lower the air pressure. There is less air above you to push the air down (which would increase the air pressure). This is why climbers on Everest use oxygen tanks - the air outside is too thin at the summit for them to breathe normally. Human bodies are used to air pressure. The air pressure in our lungs, ears and stomachs is the same as the air pressure outside of our bodies, which ensures that we don’t get crushed. Our bodies are also flexible enough to cope when the inside and outside pressures aren’t exactly the same. Airplanes need pressurized cabins to compensate for the lower air pressure at high altitudes. Despite this “pretend” atmosphere, the air pressure inside an plane is not the same as at sea level. You might have noticed that if you drink from a plastic bottle during a flight and put the lid back on, when you land the bottle will be crushed. This is because the air in the bottle is at the lower pressure of the airplane cabin and it can’t withstand the higher air pressure at ground level.
You’ve probably also noticed that your ears pop during the take off or landing of a flight. This is caused by the difference in air pressure on either side of your ear drums and the only way to equalize the pressures is to yawn, chew some gum or breathing out while holding your nose.
Try it Yourself: